Did you know? If your clinic treats patients with limited English proficiency (LEP), providing consent forms in their preferred language isn’t optional - it’s legally required. Federal laws like Title VI of the Civil Rights Act and Section 1557 of the Affordable Care Act mandate that healthcare providers ensure “meaningful access” for LEP individuals. This includes offering multilingual consent forms and translation services, all at no cost to the patient.
Here’s what you need to know:
- Why it matters: Language barriers can lead to medical errors, lower patient satisfaction, and legal risks, including malpractice claims and HIPAA violations.
- What’s required: Consent forms must be accurate, clear, and in a language the patient understands. Certified translators are essential to meet these standards.
- How to comply: Use professional translation services, train staff to handle consent discussions, and consider digital intake tools to streamline the process.
Failing to meet these requirements could result in hefty fines, civil rights violations, or harm to your patients. Multilingual consent forms aren’t just about following the law - they’re key to providing safe, effective care.
What Informed Consent Means in Aesthetics and Wellness
8 Required Elements of Medical Informed Consent Forms
Informed consent is a continuous conversation between a provider and their patient, grounded in the patient’s right to make decisions about their own body. This dialogue is based on the principle of "respect for persons", which recognizes individuals as autonomous decision-makers capable of choosing what happens to them. In aesthetics and wellness settings - whether it’s Botox, dermal fillers, or laser treatments - this means giving patients all the information they need to make educated choices about their care.
The process includes three key steps: sharing clear details about the procedure, ensuring the patient fully understands that information, and supporting a voluntary decision free from pressure or undue influence. It’s both a moral and legal obligation. Federal regulations specify that the information must be communicated in a way the individual can comprehend, and consent must be obtained in advance and properly documented to meet legal standards.
"The principle of respect for persons requires that individuals be treated as autonomous agents and that the rights and welfare of persons with diminished autonomy be appropriately protected." – HHS.gov
Informed consent isn’t just about signing a form; it’s an ongoing conversation. Documentation is part of the process, but the dialogue should continue throughout the treatment relationship. This ensures patients can ask questions at any time and that practitioners update them as new risks or benefits arise. These principles shape the essential elements that must be included in every informed consent document.
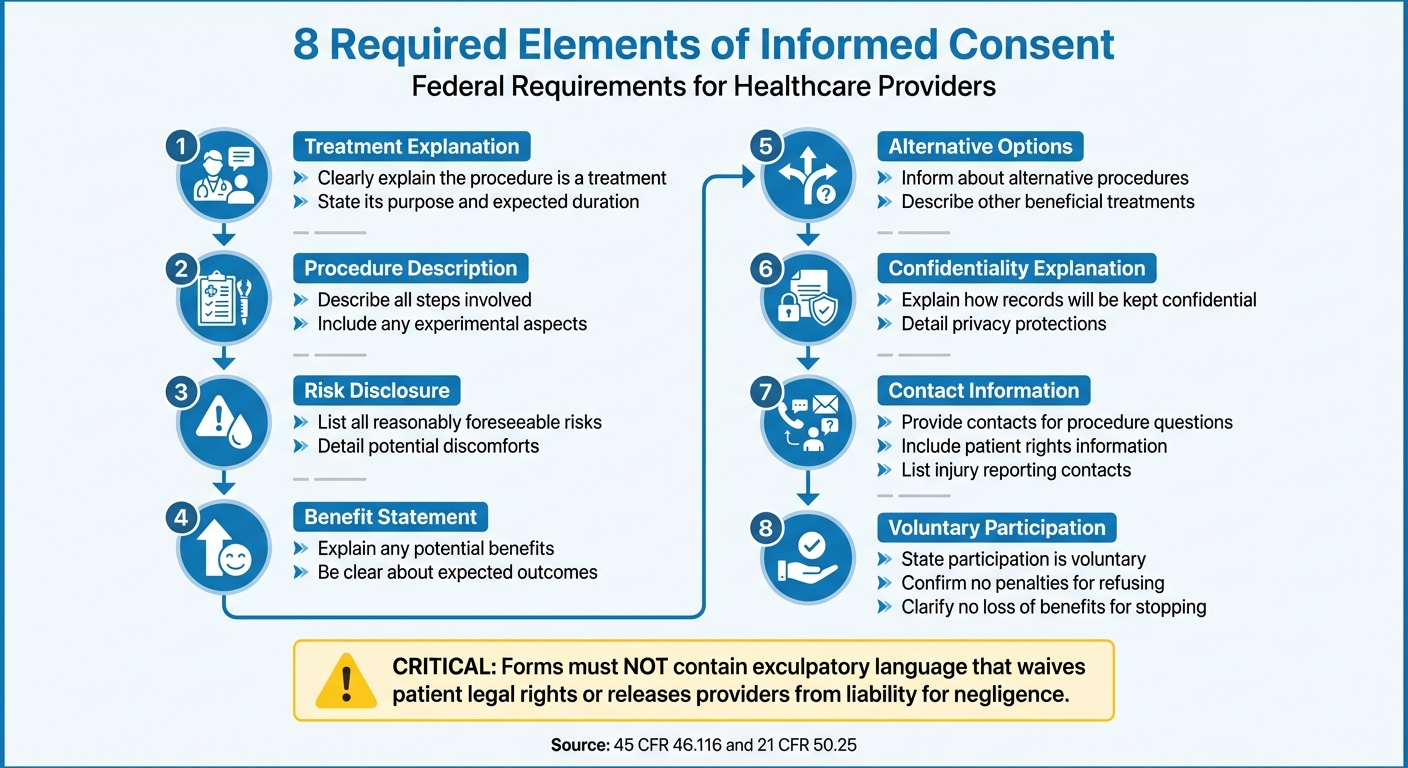
Required Components of Informed Consent
Federal regulations (45 CFR 46.116 and 21 CFR 50.25) outline eight essential elements that every consent form must include. These forms should clearly explain that the procedure is a treatment, its purpose, and how long it is expected to take. They must also describe the steps involved, including any experimental aspects, and disclose all reasonably foreseeable risks or discomforts, along with any potential benefits.
Practitioners are required to inform patients about alternative procedures or treatments that could be beneficial. Consent forms should also explain how patient records will be kept confidential and provide contact information for questions about the procedure, patient rights, or what to do in case of an injury. For remote consultations, telehealth platforms must also ensure these discussions remain private and secure. Importantly, it must be clear that participation is voluntary and that refusing or stopping treatment will not result in penalties or loss of benefits.
Depending on the procedure, additional details may be necessary. These might include information about extra costs, the consequences of withdrawing from treatment, or situations where the provider might decide to end the treatment.
One critical rule: consent forms must not contain exculpatory language. In other words, they cannot include wording that waives the patient’s legal rights or releases the provider, practice, or institution from liability for negligence.
Why Written Consent Forms Are Legally Required
Written consent forms serve multiple purposes beyond meeting legal requirements. They provide patients with a detailed "take-home" summary of the procedure and include contact information for any follow-up questions. These documents also create a record of the patient’s voluntary agreement, acknowledging specific risks and the details of the procedure.
Importantly, written consent ensures that patients have enough time to consider their decision, reducing the risk of coercion or undue influence. To support this, it’s best to avoid obtaining consent immediately before a procedure, when patients might feel rushed or pressured. Giving them time to review the information at their own pace helps ensure that their decision is genuinely informed.
sbb-itb-02f5876
Legal Requirements for Multilingual Consent Forms
Federal Laws: Title VI and Section 1557
Under Title VI of the Civil Rights Act of 1964 and Section 1557 of the Affordable Care Act, healthcare providers are required to ensure language access for individuals with Limited English Proficiency (LEP). Section 1557 specifically prohibits national origin discrimination in programs funded by federal dollars. This means that any aesthetics or wellness clinic accepting Medicaid payments or participating in Health Insurance Marketplaces must comply with these regulations.
To meet these requirements, covered entities must take "reasonable steps" to provide meaningful access for LEP individuals. This includes offering oral interpretation services and written translations of critical documents like consent forms.
"The prohibition on national origin discrimination requires covered entities to take reasonable steps to provide meaningful access to each individual with limited English proficiency who is eligible to be served or likely to be encountered." – Office for Civil Rights (OCR), HHS
Clinics are also required to display notices informing patients of their communication rights, accompanied by taglines in the top 15 languages spoken by LEP populations in their state. To assist with compliance, the Office for Civil Rights has made sample notices and taglines available in 64 languages. Importantly, these services must be provided at no cost to patients, and clinics are prohibited from using unqualified staff or unreliable video remote interpreting services.
These federal guidelines create a baseline for language access, leaving room for states to implement additional requirements.
State-Level Requirements
While federal laws set the groundwork, state-specific rules can vary significantly depending on local LEP demographics. For instance, a clinic in California may need to provide materials in different languages compared to one in Texas or Florida, based on the most commonly spoken languages in their regions.
To remain compliant, clinics must monitor the linguistic needs of their state’s population and adapt accordingly. Federal regulations allow for flexibility, considering factors like the nature of the healthcare program and the specific communication context. This means aesthetics and wellness clinics must evaluate their patient demographics and tailor their language access strategies to ensure they meet both federal and state requirements effectively.
Translation and Documentation Standards
Using Certified Medical Translators
Federal and state guidelines emphasize the importance of clear and accurate translation in medical settings. According to federal regulations, informed consent must be presented in a language the patient can understand. This means professional translation services are not just recommended - they are legally required. Certified translations include a signed statement from a qualified professional verifying the accuracy and completeness of the document. Considering that over 25 million U.S. residents have limited English proficiency, the need for precise medical translations cannot be overstated. Translators must have a solid grasp of medical terminology and cultural nuances to avoid potentially dangerous errors, such as incorrect medication dosages or misinterpreted treatment plans. Additionally, HIPAA mandates that any non-English patient information is translated accurately to maintain both confidentiality and compliance.
"In the medical field, the stakes are particularly high, as translation errors or misunderstandings can have serious consequences for patient care and safety." - MotaWord
In research and medical procedures, clinics often need to submit a Certificate of Translation along with the translated document for Institutional Review Board (IRB) approval. To save time and resources, it's generally best to finalize the English version of the consent form before starting translations. This minimizes the risk of needing costly re-translations if changes are made later. For clinics using an external translation service, a business associate agreement is usually required to meet HIPAA Privacy Rules. Certified translations not only ensure accuracy but also provide a legal safeguard through clear signature protocols.
Signature Requirements and Short-Form Procedures
When a full translation isn't practical, specific signature protocols and short-form consent procedures help maintain patient understanding and legal compliance. In some cases, a short-form consent process can be used for patients who speak a different language. This involves an oral explanation of the consent information, paired with a brief written document in the patient’s language confirming that the consent elements were presented orally. The English version of the full consent form serves as a summary document.
The signature process for short-form consent requires multiple steps. The patient signs the short form, while the witness signs both the short form and the English summary. Additionally, the person obtaining consent must sign the English summary. The witness, who must be fluent in both English and the patient’s language, should not be affiliated with the procedure. In some cases, the translator assisting with the consent process can also serve as the witness.
"The short form document should be signed by the subject (or the subject's legally authorized representative); the summary (i.e., the English language informed consent document) should be signed by the person obtaining consent... and the short form document and the summary should be signed by the witness." - Melody H. Lin, Ph.D., Director, Division of Human Subject Protections, OPRR
Before use, all foreign-language consent documents, including short forms, must receive IRB approval. Patients should also be given copies of both the signed short form and the English summary. These documents can be securely shared and stored within a patient portal for easy access. It’s crucial to avoid relying on minors or unqualified adults - such as family members or friends - for interpretation or consent facilitation unless it’s an emergency involving an immediate threat to safety.
How to Implement Multilingual Consent Forms
Creating Procedure-Specific Consent Forms
One-size-fits-all templates just don’t cut it when it comes to legal and patient care standards. Every procedure needs its own tailored consent form, clearly explaining the purpose, duration, process, risks, benefits, and available alternatives - all in straightforward, easy-to-read language. Federal regulations require that consent forms include these details to ensure patients are fully informed before agreeing to any procedure.
Here’s where simplicity matters. While the average U.S. adult reads at about an 8th-grade level, the American Medical Association suggests that patient materials should aim for a 6th-grade reading level. Swapping out medical jargon for simpler terms can go a long way. For example, use “likely results” instead of “prognosis” or “other possible options” instead of “alternative methods.” Speaking directly to the patient by using "I/me" or "you/your" language also makes consent forms more approachable.
A great example of this approach comes from a 2019 study at the Medical University of Gdańsk. Researchers simplified a Polish-language plastic surgery consent form originally written for people with a university-level education. Using "Jasnopis" linguistic software, they reworked the form to be understandable for individuals with a 4th–6th grade education. The results? Seventy-eight percent of participants found the revised form completely understandable, compared to the original version, which was labeled as overly complex or professional.
Training Staff and Conducting Consent Discussions
Even the best-translated forms won’t work if staff aren’t trained to use them effectively. It’s crucial to train team members to engage patients in ongoing, clear consent discussions. If a consent conversation happens in a specific language - like Spanish - the patient should sign the corresponding translated form. The person obtaining consent should also sign this same version to document the interaction.
"Informed consent is an ongoing, interactive process that helps participants make educated decisions about joining or continuing a study." – Language Scientific
To confirm patient understanding, staff can ask patients to explain the procedure back to them. This step helps ensure clarity and avoids misunderstandings. Additionally, professional interpreters should always be used for these discussions - steering clear of relying on family members or minors, except in emergencies.
Using Digital Platforms for Consent Management
Digital tools can take the consent process to the next level by making it more efficient and patient-friendly. These platforms reduce the risks tied to paper forms while improving both understanding and compliance tracking. Interactive features like diagrams, videos, and audio explanations in multiple languages allow patients to digest complex information at their own pace. And since about half of all online traffic comes from smartphones, mobile-friendly forms are a must.
HIPAA-compliant platforms like Prospyr make it easy to track every step of the consent process - form views, edits, and signatures are all logged with timestamps. These systems ensure patients always sign the latest version of the form, meeting legal requirements. Plus, under the federal E-Sign Act, electronic signatures are just as valid as traditional ones, as long as they meet verification standards. For remote signing, tools like ID checks or security questions can confirm the signer’s identity.
Digital consent tools also save time. Paper forms often take more than 20 minutes to complete, but electronic systems streamline the process. Patients can even review and sign forms at home before their appointment, reducing the rushed feeling that often comes with in-office paperwork. And when updates are needed, digital platforms notify patients instantly and ensure they’re signing the most current version of the document.
Conclusion
Multilingual consent forms are essential for safeguarding your practice while ensuring all patients can access and understand their care. Federal laws like Section 1557 of the Affordable Care Act and Title VI of the Civil Rights Act mandate that providers receiving federal funding offer meaningful access to patients with limited English proficiency. This includes providing free language assistance services and consent forms written in clear, straightforward language.
Language barriers can lead to serious consequences, such as higher risks of medical errors, extended hospital stays, and reduced patient satisfaction. Noncompliance can result in steep legal and financial penalties, including civil rights discrimination claims, HIPAA violations, and medical malpractice lawsuits. To navigate these strict requirements, having well-defined strategies is crucial.
Always use certified medical translators instead of relying on bilingual staff or family members, and secure Business Associate Agreements with translation providers to protect patient confidentiality. Post nondiscrimination notices and language assistance taglines in at least the top 15 languages spoken in your state. If a consent discussion takes place in a specific language, ensure the patient signs the corresponding translated consent form.
Incorporating digital consent tools can simplify compliance while improving clinic efficiency. These platforms track every action - from document views to signatures - with timestamps, making it easier to meet regulatory standards and enhance the patient experience. Tools like Prospyr enable aesthetics and wellness clinics to handle multilingual consent effortlessly. By prioritizing multilingual consent forms, you build trust, minimize risks, and empower patients to make informed decisions about their care.
FAQs
What are the risks of not providing consent forms in a patient's preferred language?
Not providing consent forms in a language the patient understands can lead to serious legal and financial repercussions. It may breach HIPAA regulations and civil rights laws, particularly 45 CFR §46.116/§46.117, which mandate clear communication to ensure patients can give informed consent.
Failing to comply can result in enforcement actions, revocation of funding or licensure, and civil monetary penalties ranging from $137 to $1,500,000 per violation. Beyond the legal consequences, it can damage patient trust and tarnish your clinic's reputation. Offering multilingual consent forms isn't just about compliance - it's about maintaining ethical standards and fostering confidence in your practice.
How can clinics ensure translated consent forms are accurate and compliant?
Clinics can maintain accuracy and meet compliance standards for multilingual consent forms by following a structured translation process. Begin by hiring a professional translator who specializes in medical and legal terminology. To ensure the translation is accurate, request a Certificate of Translation, which verifies that the translated document faithfully reflects the original content. Afterward, have a bilingual clinician or legal expert review the translation to confirm that all clinical and regulatory details are properly preserved. Adding a back-translation step - where a different translator converts the document back into English - can help spot any inconsistencies or errors.
Once the translation is finalized, submit it to your clinic’s compliance office or Institutional Review Board (IRB) for approval, ensuring it aligns with regulations such as 45 CFR §46.116. Keep detailed records of the entire process, retain the certificate, and securely store the approved version in a HIPAA-compliant system. Tools like Prospyr can make this process smoother by integrating digital forms with secure storage and version tracking, allowing clinics to manage accuracy and compliance more effectively.
How can clinics effectively implement digital consent forms?
To implement digital consent forms effectively, clinics must ensure they meet all legal requirements. These forms should clearly include the patient’s name, the intended purpose of use, and a secure electronic signature. Using a HIPAA-compliant platform is key to managing these forms securely. Features like data encryption, role-based access, and adherence to privacy regulations help protect sensitive patient information.
Offering multilingual consent forms is another important step, especially for clinics serving diverse communities. Certified translation services or skilled interpreters can ensure the forms are accurate and accessible to all patients. Pair this with e-signatures that include audit trails to create a secure, trackable consent process.
For better organization, integrate these forms directly with your electronic medical records (EMR) system. This ensures they are easy to access when needed. Regular staff training on privacy standards and conducting periodic security audits will help maintain compliance and streamline the workflow.



